
** Cough Syrup Exporters Mandated to Undergo Product Testing at Government Labs from June 1**
In a recent development aimed at prioritizing consumer safety and maintaining high-quality standards, the government has issued a notification, DGFT Notification No. 06/2023, mandating all cough syrup exporters to undergo compulsory product testing at government laboratories. Effective from June 1, this new requirement aims to ensure that the cough syrups being exported are safe and reliable.
As per the DGFT notification, exporters must send their cough syrup samples to designated government labs for thorough testing. These labs have advanced equipment and expert staff who will carefully examine the samples. The testing process will evaluate the strength, purity, and compliance of the cough syrups with quality standards and regulations.
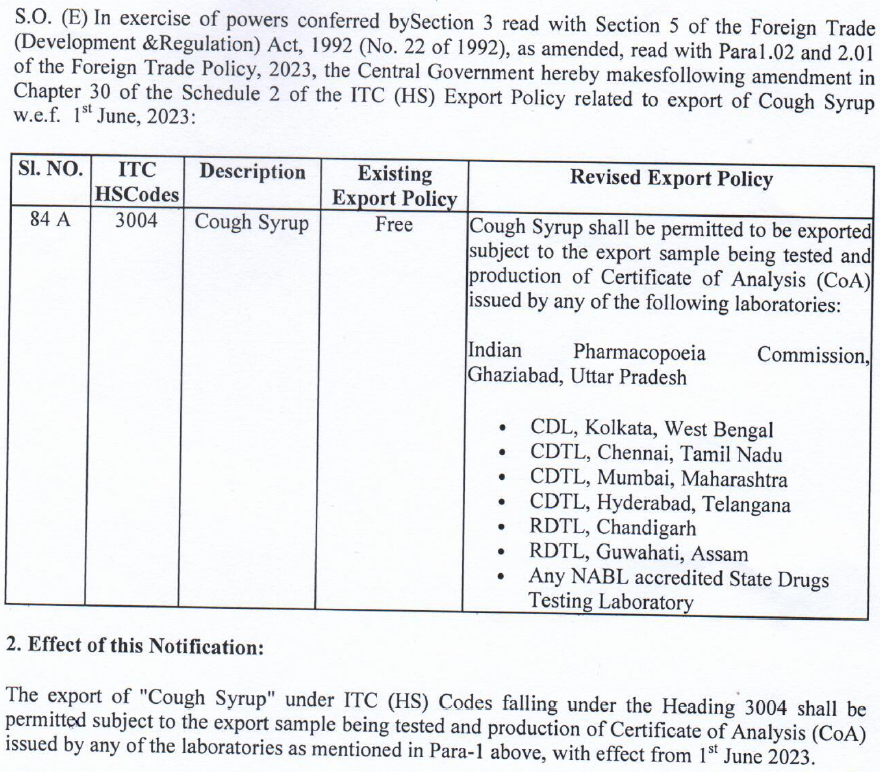
To comply with the new regulation, exporters need to ensure the timely transportation of their cough syrup samples to the government labs. It is crucial to follow these testing requirements to avoid penalties or potential suspension of export privileges.
During this transition period, the Ministry of Health and the Ministry of Commerce will provide extensive support to the exporters. They will offer guidance on testing procedures, required documentation, and associated fees. Exporters are advised to review the notification and seek clarification from the authorities if needed.
The government’s primary objective in implementing this regulation is to safeguard public health and maintain the pharmaceutical industry’s reputation. By ensuring that the exported cough syrups meet rigorous safety and quality standards, the government aims to boost consumer trust and enhance the competitiveness of the industry on a global scale
Cough syrup exporters are urged to take immediate action and comply with the testing requirements outlined in the DGFT Notification No. 06/2023. This proactive step will help ensure the safety and quality of cough syrup exports and contribute to the overall credibility of the pharmaceutical sector.
Stay updated with further developments as the government continues its efforts to enforce stringent quality control measures and protect consumer interests.
