
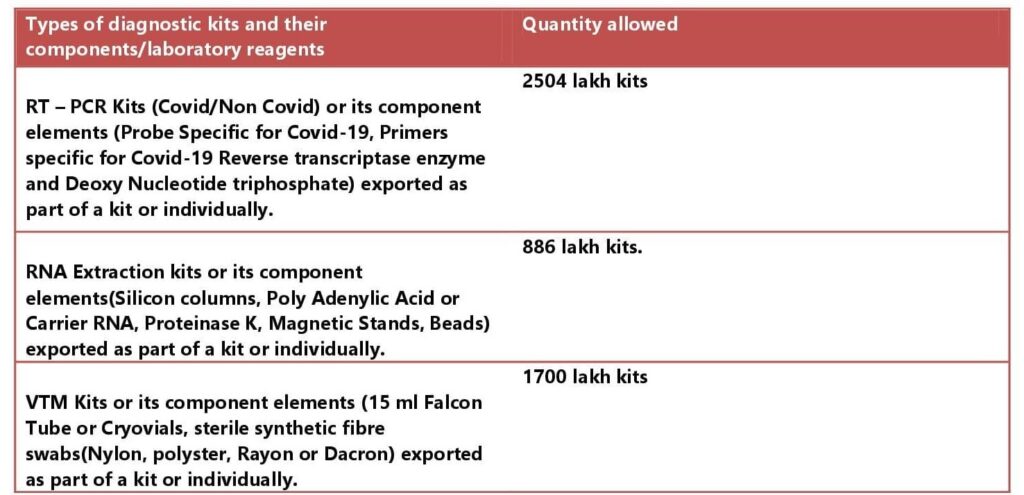
As per the trade notice, 15/2021-22 Dated 9th August, the Quota for export of the following types of diagnostic kits and their components/laboratory reagents has been fixed for the month of July, August, and September 2021 by the DGFT –
Application for Export License to export the above Restricted items (Non-SCOMET):-
- Apply online by navigating to the DGFT website (https://www.dgft.gov.in) -> Select Services -> Click on Export Management Systems -> License for Restricted Exports
- Fill out the mandatory details and attach the documents.
(To check application procedure for Import Authorisation/License to import duty-free raw materials click on – Advance Authorisation Scheme )
Important points while applying for the License:-
- Export License would be issued from DGFT Head Quarter (DGFT Delhi).
- There is no need to submit a hard copy of the application.
- Application has to be done for the above quantities from 10th August to 17th August 2021.
- The validity of the license would be 6 months only.
- Documents would be duly self-attested by the authorized person of the firm.
- The incomplete application would not get the allocation.
- Application through the mail wouldn’t be accepted.
- Application outside the timeline will not be considered.
Eligibility Criteria for consideration of the application:-
- Documentary proof of manufacturing “VTM/RNA extraction kits/RT PCR Kits,
- Copy of Purchase Order/Invoice
- Valid IEC Copy
- Undertaking duly signed by the authorized signatory in the company letterhead to be submitted by the manufacturer certifying that as of date, all domestic commitments/orders have been fulfilled.
